

On a spring day in 1958, neurophysiologist Torsten Wiesel joined his postdoctoral mentor, Stephen Kuffler, for their usual lunch meeting at a cafeteria on the campus of Johns Hopkins University. On this particular day, they were joined by David Hubel, a neurophysiologist and new postdoctoral hire in the lab. Kuffler, who made pioneering discoveries about the activity of neurons in the retina, spoke with the duo about ambitious plans to explore the brain in a way that had never been done before. Wiesel, now 100 years old, vividly recalled the meeting, noting, “I knew David from his work. I was very pleased to have a new collaborator.” What was originally meant to be a one-year project turned into a decades long collaboration that would win Wiesel and Hubel a Nobel Prize and revolutionize our understanding of the brain.
A Journey into the Brain
The 1950s were a relatively rudimentary era for experimental neurophysiology. Recording the electrical activity of neurons wasn’t uncommon, but the methods often demanded considerable patience and persistence. Glass micropipettes fitted with a conductive material, such as a salt solution, were a significant innovation that enabled researchers to record the first electrical pulses directly from neurons in a giant squid.1 But they were finicky pieces of equipment that tended to damage neurons. Furthermore, they worked reasonably well for recording the electrical variations that arise from the collective activity of hundreds or thousands of neurons in the brain, but Hubel and Wiesel had a loftier goal; they wanted to record the electrical impulses from single neurons to determine how different neurons respond to visual stimuli. In doing this, they had a chance to gain insights into how the brain processes information.
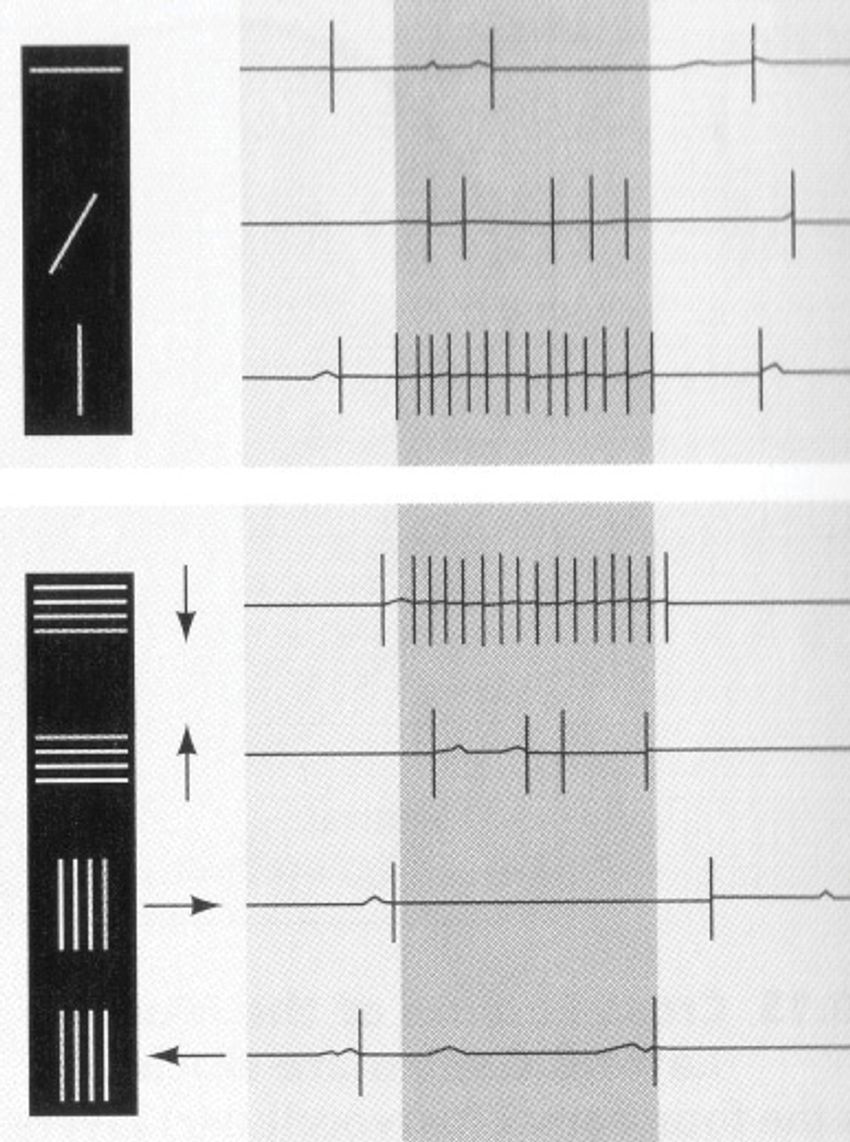
Neurophysiology experiments in the mid-20th century were often conducted with cats as they had a manageable size and a comparative neuroanatomy that researchers felt could provide insights into the workings of the human brain. With micropipettes, it was possible to record activity in a cat’s retina while the animal was under anesthesia. Using these tools, Kuffler had recorded vigorous activity in neurons called retinal ganglion cells while cats viewed small, circular shadows that appeared on a white background. As soon as the shadow was displayed in a surrounding region, neuronal firing ceased nearly completely. The spot caused both neuronal excitation and inhibition depending on its location in space.
Hubel and Wiesel found that neurons in the visual cortex show orientation selectivity.
Jimmierock
Hubel and Wiesel initially demonstrated that, further along the visual pathway in the cortex, cells in a brain region called the geniculate nucleus also respond to spots of light. They wanted to build on these discoveries by making pioneering recordings from a neuron in the visual cortex that received direct input from geniculate cells. But this brain region posed a challenge: Maintaining stable recording from a single neuron using micropipettes was difficult, necessitating a more stable solution.
Thankfully, during his previous position at Walter Reed Army Institute of Research, Hubel—guided by a department machinist skilled in metallurgy—created a tungsten electrode.2 This sturdy tool allowed for stable recordings of individual neurons, or in neurophysiological parlance, single units. Reflecting on the technique, Wiesel said, “It was much better for isolating single units than the older electrodes. With a tungsten electrode, you could record from a cell for hours without interference in the health of the cell.” For the first time, they could now observe whether any patterns in the brain’s cortical responses corresponded to visual stimuli presented to the cat. Essentially, they could begin cracking a neuron’s code.
Playing Cat and Mouse with Neurons
And so began the recording sessions. Despite the sophistication of Hubel and Wiesel’s new technique, systematically exploring responses in individual neurons in real time—while maintaining electrode stability using only analog equipment—required long hours. To maximize efficiency, the duo prioritized collecting as much data as possible in one go. This meant that the sessions often went late into the night, if not well into the morning.
Even with their immense effort and commitment, it was a very trying time. Nothing seemed to work. They struggled to record consistent, strong neuronal firing that could indicate meaningful variations in response to the visual stimuli presented to the cats. After weeks of probing neurons, they didn’t discover anything particularly meaningful. But they pressed on.

Wiesel (left) and Hubel (right) joined Harvard University, although at different times, where they continued studying sensory processing.
Joe Wrinn
During another attempt, they managed to achieve a stable recording, but once again, the neuron wasn’t responding to any visual stimuli presented to the cat. Four hours into what would become a nine-hour recording session, they had only captured a handful of small, inconsistent responses. But just as the day appeared to be unfolding uneventfully, everything changed.
Suddenly, as they removed the glass projector slide to swap it out for a new one, something extraordinary happened. The audiomonitor “went off like a machine gun!,” recalled Hubel in his 1981 Nobel Prize lecture. The neuron was firing at a rate unparalleled to any they had encountered. They were ecstatic, but their excitement was pierced with anxiety. They had done this swap countless times in previous recording events. What exactly caused the neuron to fire so vigorously this time? Moreover, could they elicit the same response again?
After carefully retracing their steps, they realized what excited the cell had nothing to do with the spot they were showing the cat. Rather, it was the dim, but clear, shadow created by the edge of the projector slide that got the cell firing. It seemed to respond to a contour rather than a spot. As they changed the angle of this shadow line and displayed it again and again, the cell became less and less activated. This suggested that the neuron’s firing was selective for the contour when it was in a specific orientation—the exact angle they happened to present to that cat by chance.
They were thrilled. Wiesel recalled rushing down the halls to rally their colleagues to join in the celebration. After so many lengthy recording sessions, they finally found the signal.
Expanding the Visual Field
The data from Hubel and Wiesel’s fateful recording session implied that the cortex was processing the information it received from the retina and the geniculate nucleus—a major breakthrough at the time. And they achieved this with a relatively simple slide projector considering there was more advanced technology available at the time. Charles Gilbert, a neurobiologist at The Rockefeller University and former student of Wiesel’s, said, “It was funny because there were other labs at the time that had devised very elaborate, complex instrumentation. But it wasn’t until David and Torsten were fiddling around with a much cruder basis of instrumentation that they actually discovered this property of orientation selectivity, which was the first major discovery of something that emerged in the cortex that wasn’t a property of antecedent stages and visual pathway.”

Hubel and Wiesel’s discoveries on sensory processing won them a Nobel Prize in 1981.
Joe Wrinn
But the question remained: What exactly was the cortex doing with the information it received? Wiesel and Hubel had essentially discovered something akin to an unfinished puzzle, and they weren’t sure how the remaining pieces fit together.
They changed their stimuli from spots to bars that resembled the shadow line that triggered the first cell. After weeks of testing how cells responded to the new shape, a logical pattern began to slowly emerge. In a manner similar to the retinal ganglion and geniculate cells, cortical cells also responded to stimuli in distinct regions of visual space, their activity being either excited or inhibited by a bar appearing in that region. But instead of having a circular response zone, the cortical neurons activated vigorously when the bars appeared in small, elongated areas of the visual field.3 However, they were inhibited when the bar entered parallel, neighboring regions of similar shape.
As they examined the unsolved puzzle that lay before them, they realized that individual cells in the visual cortex appeared to be combining inputs from several geniculate cells that were sensitive to projected spots in overlapping adjacent regions in the visual field. They surmised that these overlapping zones formed the elongated excitatory and inhibitory areas.
“They discovered the first step in information processing that the cortex carries out. This was big,” said Margaret Livingstone, a neurobiologist at Harvard University who collaborated with Hubel and Wiesel during these early years “They saw that the cortex combines the spot-like selectivity of geniculate inputs in to contour-selective receptive fields, which goes a long way towards understanding how we perceive objects.”
Visual Development Has Critical Periods
Hubel and Wiesel’s discoveries didn’t end there. They found other cortical cell types that fired vigorously to a moving contour, which they believed connected with the cells that they previously identified. Over the following months, they discovered numerous cells with other distinct response characteristics that would eventually lead to the discovery of neurons that appear highly selective for complex visual features like hands, tools or even words.4
Wiesel became increasingly interested in exploring whether these visual cortical cells harbored such distinct properties from birth or if visual experiences played a role in their development. He considered children with congenital cataracts, who often continue to have visual deficits after cataract removal. If they had matured with cataracts beyond a certain age, was the brain unable to rewire itself following the corrective eye surgery? It was a debate of nature versus nurture.
They discovered the first step in information processing that the cortex carries out. This was big.
—Margaret Livingstone, Harvard University
Throughout the 1960s and 1970s, Hubel and Wiesel continued their partnership, focusing on how visual input affects development, now using monkeys.5 To do this, they tuned the inputs that each eye received at different developmental stages, occasionally covering one eye to assess the role of visual experience before and after maturity. After weeks of deprivation, they observed that developing cortical cells responded most strongly to visual stimuli presented to the eye that was open during development. This was further supported by histological analyses that revealed more neuronal connections to the open eye. The researchers were astonished to find that if the animal was still young and they switched which eye was covered, the connections could be redirected to the other open eye. However, if the animal had matured beyond a certain age, it was too late: The connections had become mostly permanent.
Hubel and Wiesel demonstrated that the visual cortex is organized into areas with increasingly specialized functions as information is processed along connections from the retina into cortical regions. They also found that the development of this complex visual system depends on experience before adulthood. Building on these discoveries, Gilbert and Wiesel determined that neurons could not only change their function after days or weeks of sensory deprivation but also exhibit different functions at various times under normal, healthy conditions. Gilbert explained that through their research, “Neurons are thought of more as adaptive processors, assuming different functions at different times, and so the actual information conveyed by a particular neuron at a particular time changes on a moment-to-moment basis.” They attributed these changes to complex patterns of long-range connections within the visual cortex that connected areas both further and closer to the eye. These horizontal connections are highly dynamic, adjusting to different environments and changing as an animal learns.6
Hubel and Wiesel were awarded a Nobel Prize for their research in 1981, which they shared with California Institute of Technology neuroscientist Roger Sperry. Reflecting on his eminent career, marked with challenges, disappointments, and triumphs, Wiesel simply said, “It was like exploring nature. You have to try different ways and it’s not that you know what you will find. It’s that you have to be humble. You have to listen to nature and see what you find.”
Source link




